Abstract
Introduction
The DAWN study (NCT01779791) evaluated efficacy and safety of the Bruton's tyrosine kinase (BTK) inhibitor ibrutinib as monotherapy in relapsed/refractory (R/R) follicular lymphoma (FL) patients (Gopal AK, et al. J Clin Oncol. doi: 10.1200/JCO.2017.76.8853). The overall response rate (ORR) for ibrutinib was 20.9% (95% confidence interval, 13.7-29.7), not meeting the primary end point; however, responders experienced a long duration of response with a median of 19.4 months. We present the results of a biomarker investigation performed on samples from the DAWN study to determine whether somatic mutations could be used to identify FL patients who responded to ibrutinib.
Methods
DAWN was a multicenter, single-arm, phase 2 study of ibrutinib (560 mg QD) in FL pts with ≥ 2 prior lines of therapy and progressive disease (PD) ≤ 12 months after chemoimmunotherapy (CIT). The primary end point was ORR (complete response [CR] + partial response [PR]). Whole exome sequencing was performed on formalin-fixed, paraffin-embedded tumor samples from 83 patients with available response data - either "responder" (CR + PR; n = 17) or "nonresponder" (stable disease [SD] + PD; n = 66) following ibrutinib treatment. Multiple filters were applied to rule out potential germline variants, and a custom panel of 1216 genes known to be involved in cancer was used for further analysis. Variants enriched in responders or nonresponders were identified using Fisher's exact test. Classifiers were built with variable numbers of genes ranked with a greedy algorithm that selected genes that would, at each iteration, allow the removal of the greatest number of nonresponders from the patient pool, while severely penalizing the removal of responders; classifier performance was assessed using 10-fold cross-validation.
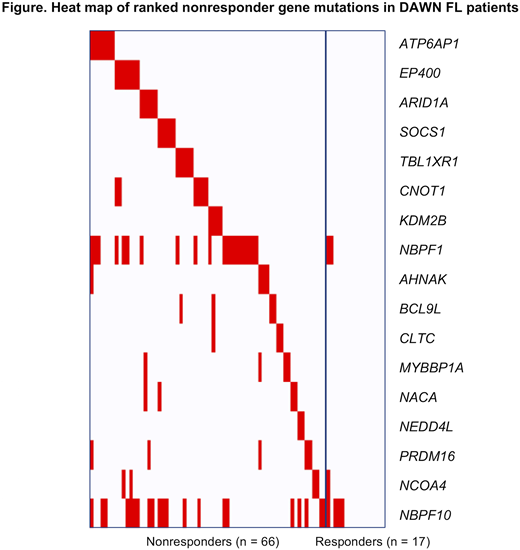
Results
The overall pattern of variant frequencies identified from the whole exome sequencing in this study was comparable to previously published studies in FL (Krysiak K, et al. Blood. 2017;129:473-483). As there were many more nonresponders than responders in this study, univariate analysis yielded mostly variants significantly enriched in ibrutinib responders but in very low numbers, eg, FANCA, HISTH1B, ANXA6, and PARP10; interestingly, 2 patients with variants in BTG1, which is considered a tumor suppressor, also responded to ibrutinib. Few nonresponder genes were identified in univariate analysis, including NBPF1, ATP6AP1, EP400, and CNOT1; mutations in these genes may activate pathways that bypass BTK, including the mTOR and JAK/STAT pathways. From the selected panel, a 17-gene classifier was developed (Figure) that included variants in ATP6AP1, EP400, ARID1A, SOCS1, TBL1XR1, CNOT1, and KDM2B. Many of these mutated genes have previously been associated with a poor prognosis in cancers, including FL; the biological functions of these genes involve transcription, cell cycle, DNA repair, cell adhesion, protein processing, and transport. Notably, few significant variants emerged that are directly involved in the BTK pathway, unlike previous reports in diffuse large B-cell lymphoma, although, as mentioned above, a few may represent bypass mechanisms to this pathway.
Conclusions
Mutational analysis of genes in patients from the phase 2 DAWN trial yielded insights into the mechanism of ibrutinib response and resistance in R/R FL. The genes involved in this mechanism demonstrate a large variety of biological functions and show that ibrutinib activity in FL may extend beyond the BTK-NF-kB pathway to gene and protein regulation, DNA repair, adhesion, and other cellular and microenvironmental processes. We have shown previously that its immune activity is also an important mechanism of action in FL (Gopal 2018). A gene-based classifier has been developed that we hypothesize may prove useful in enriching for ibrutinib response in FL; however, this will need to be validated in other data sets.
Funding source
Sponsored by Janssen Research & Development, LLC. Writing assistance was provided by Jill See of PAREXEL and funded by Janssen Global Services, LLC.
Balasubramanian:Janssen Research & Development: Employment, Equity Ownership. Hodkinson:Janssen Research & Development: Employment. Schuster:Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Research Funding; Dava Oncology: Consultancy, Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees. Fowler:Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Trotman:Takeda: Other: unremunerated advisory role; Celgene: Other: unremunerated advisory role; Roche: Other: unremunerated advisory role; Janssen: Consultancy, Research Funding. Hess:CTI: Research Funding; Celgene: Consultancy, Honoraria, Other: travel expenses, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cheson:AbbVie, Roche/Genentech, Pharmacyclics, Acerta, TG Therapeutics: Consultancy. Schaffer:Janssen Research & Development: Employment, Equity Ownership. Wang:Janssen Research & Development: Employment. Deshpande:Janssen Research & Development: Employment. Vermeulen:Janssen Research & Development: Employment, Equity Ownership. Salles:Gilead: Honoraria, Other: Advisory Board; Amgen: Honoraria; Servier: Honoraria, Other: Advisory Board; Merck: Honoraria; BMS: Honoraria, Other: Advisory Board; Servier: Honoraria; Morphosys: Honoraria; Pfizer: Honoraria; Epizyme: Honoraria; Celgene: Honoraria, Other: Advisory Board, Research Funding; Janssen: Honoraria, Other: Advisory Board; Novartis: Consultancy, Honoraria; Takeda: Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; Acerta: Honoraria. Gopal:Teva: Research Funding; Brim: Consultancy; Asana: Consultancy; Janssen: Consultancy, Research Funding; Takeda: Research Funding; Pfizer: Research Funding; Gilead: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Merck: Research Funding; Aptevo: Consultancy; BMS: Research Funding; Incyte: Consultancy; Spectrum: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal